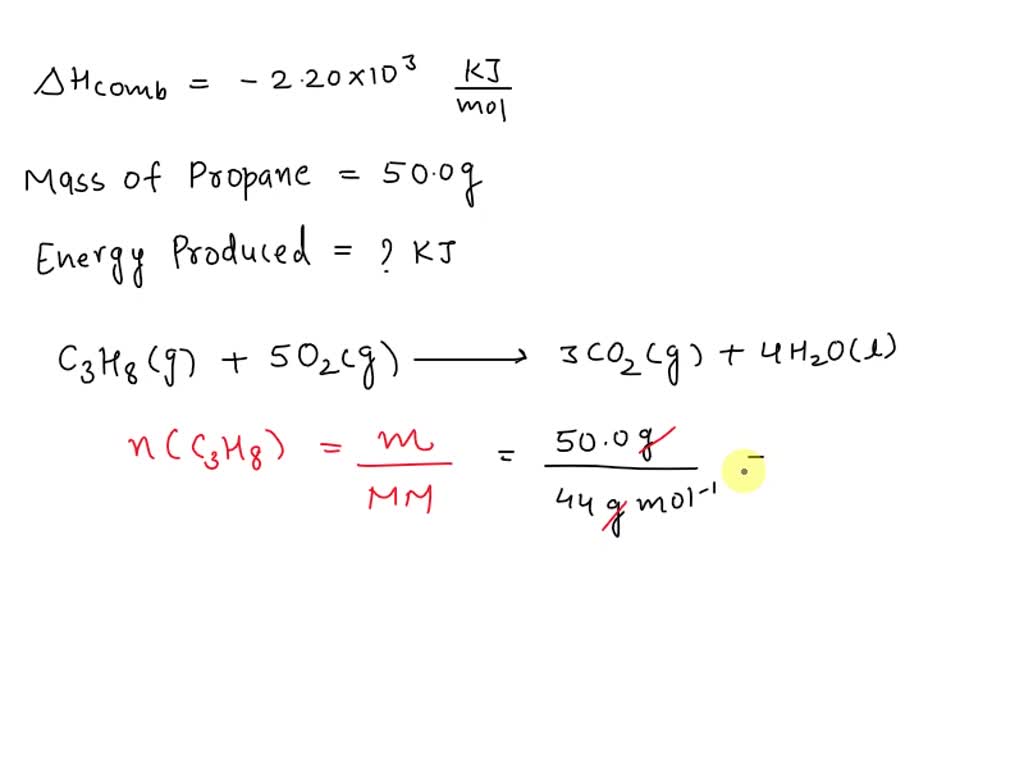
The standard heat of combustion of propane is -2220.1 kJ/mol. The standard heat of vaporization of liquid water is 44 kJ/mol. What is the triangle H^{o} of the reaction :C_{3} H_{8} (g)+
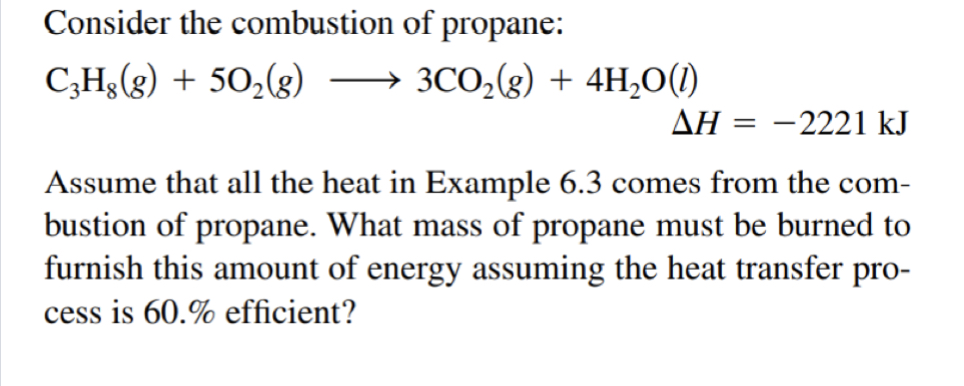
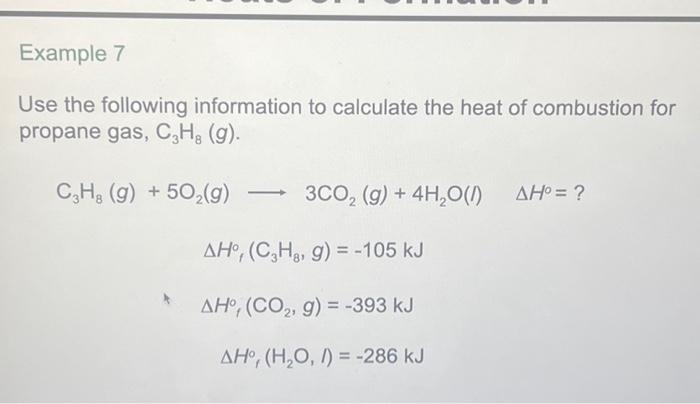
SOLVED: Consider the combustion of propane: C3H8(g)+5 O2(g) ⟶ 3 CO2(g)+4 H2O(l) Δ H=-2221 kJ Assume that all the heat in Example 6.3 comes from the combustion of propane. What mass of
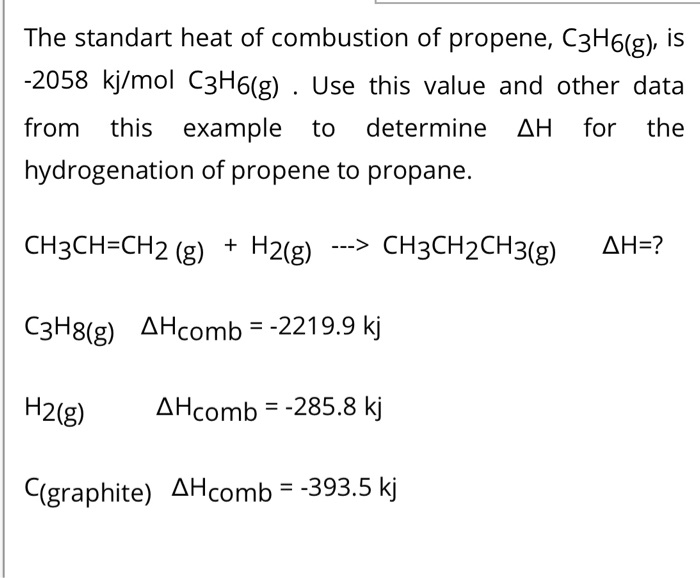
SOLVED: The standard heat of combustion of propene, C3H6(g), is -2058 kJ/mol C3H6(g). Use this value and other data from this example to determine ΔH for the hydrogenation of propene to propane:

60. If heat of formation of mathrm{C}_{3} mathrm{H}_{8}, mathrm{CO}_{2} and mathrm{H}_{2} mathrm{O} are -38.2,-94.1 and -48.2 kcal respectively. Then calculate heat of combustion of propane: (1) -423.3 mathrm{kcal} (2) +423.3 mathrm{kcal} (3) -

SOLVED: Consider the combustion of propane: C3H8(g) + 5O2(g) â†' 3CO2(g) + 4H2O(g) ΔH = -2221 kJ Assume that all of the heat comes from the combustion of propane. Calculate ΔH when

3. The standard heat of combustion of propane is -2220.1 kJ mol-! The standard heat of varonisation of liquid water is 44.0 kJ mol-. What is AH of - CoHs (9) +502 (
Calculate the standard heat of formation of propane, if its heat of combustion is -2220.2 KJ mol^-1 , the heats of formation - Sarthaks eConnect | Largest Online Education Community

A 10.0 g sample of propane, C3H8, was combusted in a constant-volume bomb calorimeter. The total heat - Brainly.in
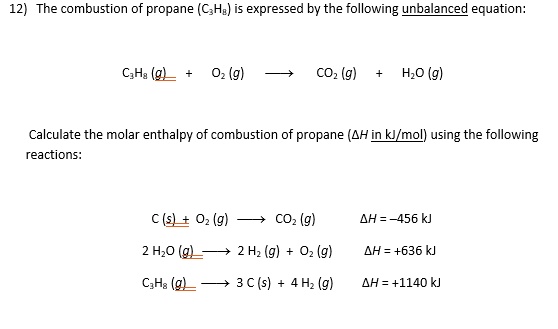
SOLVED: The combustion of propane (C3H8) is expressed by the following unbalanced equation: C3H8 (g) + O2 (g) -> CO2 (g) + H2O (g) Calculate the molar enthalpy of combustion of propane (